FDA Approves At-Home Flu Vaccine: What the Self-Administered Spray Means for You.
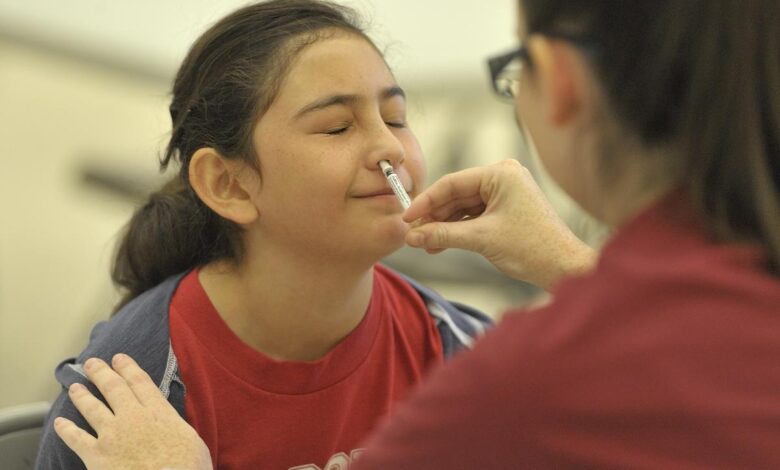
Prepare for an exhilarating leap into the realm of flu vaccination! As of September 20, the FDA has unleashed a groundbreaking innovation, granting the green light for a nasal spray flu vaccine that can be self-administered by adults or dutifully administered by caregivers to their little ones. The heralded nasal spray, known as FluMist, has lingered in the health care shadows since 2003, previously confined to the skilled hands of pharmacists and health professionals.
Curious minds, read on! Here’s what you ought to know about the audacious new nasal flu vaccine.
What is FluMist?
The illustrious nasal spray vaccine has been a player in the medical scene for over twenty years. Initially authorized for those aged 5 to 49, its reach expanded in 2007 to embrace children as young as 2. The ingenious FluMist showcases a single-dose mechanism, delivering a precise spray into each nostril.
Distinct from its syringe-wielding counterpart, the FluMist nasal spray flaunts a composition featuring what is classified as a live-attenuated flu virus. This means it carries a version of the virus that’s been toned down but not eradicated. In contrast, flu shots incorporate a dead or inactivated virus. Fear not—the nasal spray is designed to avoid inducing any influenza illness. According to the FluMist package insert, this sprightly spray has an efficacy hovering around 45%, comparable to the flu shot’s 40% to 60% range.
When can you get an at-home FluMist vaccine?
The quest for a self-administered flu vaccine has been swirling in the ether for ages. However, patience is key; the FDA has indicated that a widespread release for home use is anticipated for the 2025 flu season. Yet, for those who are keen on the nasal spray for themselves or their progeny, it’s already accessible in numerous pharmacies and health care settings—albeit under the watchful eye of a provider.
Yahoo Life reached out to the illustrious maker of FluMist, AstraZeneca, seeking clarity on the timeline for home usage. Yet, the silence lingered. One prevailing theory suggests that the delay stems from the need for collected data regarding the effectiveness of self-administration, as articulated by Dr. Davey Smith, chief of infectious diseases at UC San Diego.
Who should use it?
FluMist proudly welcomes individuals aged 2 to 49 into its fold. Dr. Smith expresses hope that home use will encourage greater vaccination rates, especially among children and those with needle-related anxieties. “Navigating our health care system is riddled with obstacles, so I pray this is one less.”
Nevertheless, eligibility isn’t a given. The criteria remain cloaked in mystery, but indications suggest that age checks and medication compatibility will be prerequisites. Notably, those aged 2 to 17 on aspirin should steer clear of FluMist due to its potential links to the rare yet serious Reye’s syndrome. Pregnant women are also advised to avoid the nasal spray, albeit the injected flu vaccine remains safe for them.
Moreover, the FDA cautionary note highlights that individuals with compromised immune systems or specific allergies ought to avoid FluMist, echoing the same advisories for other live-attenuated vaccines. The nasal spray isn’t suitable for those aged 50 or older, as the activated immune response differs and may be less robust compared to the injected version.
Are live-attenuated virus vaccines safe for most people?
Experts resoundingly affirm: Yes! The nasal spray’s live, albeit weakened, virus is crafted for cold-temperature adaptation, reproducing itself serenely in the cooler confines of the nose without venturing into the lung territory where infections loom. Dr. Paul Offit reassures that while the side effects may resemble those associated with the shot—a deluge of runny noses, nasal congestion, fleeting fevers in toddlers, and mild sore throats in adults—these effects are generally mild.
How do you give yourself FluMist?
Administering FluMist is akin to wielding your trusty allergy nasal spray, Flonase. Simply insert the nozzle, engage the plunger for a glorious blast of vaccine goodness, and repeat on the opposing nostril! Dr. Smith harbors no apprehensions about self-administration, as it’s a familiar ritual: “People have been self-spraying Flonase for eons … it’s as intuitive as it gets.” AstraZeneca’s trials reveal a staggering 100% success rate among adults administering the full dose independently.
This ease of use heralds a bright future for vaccination endeavors, particularly for children and those who balk at needles. Dr. Offit opines that FluMist’s home availability could catapult flu vaccination rates, which have been dwindling—plummeting to a dismal 50% as of last year. A hopeful horizon awaits!




