FDA Approves At-Home Flu Vaccine: What This Self-Administered Spray Means for You.
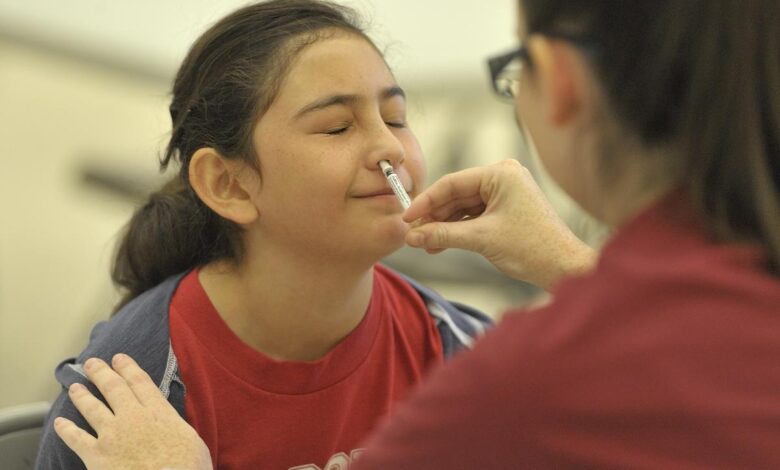
Exciting news in the realm of flu prevention! Now, you can easily protect yourself—and your little ones—against the flu with a convenient nasal spray. On September 20, the Food and Drug Administration (FDA) granted approval for a groundbreaking vaccine that can be self-administered by adults or given by caregivers. While the nasal spray known as FluMist has been around since 2003, it previously required administration by healthcare professionals like pharmacists.
Delve deeper into the nuances of this newly accessible nasal spray flu vaccine.
What exactly is FluMist?
The nasal spray vaccine has existed for over two decades, initially approved for administration to individuals ages 5 to 49 by pharmacists and healthcare providers. In 2007, its approval was broadened to encompass children as young as 2. This single-dose sprayer requires half the dose to be sprayed into each nostril.
Beyond being a spray rather than an injection, FluMist sets itself apart by utilizing a live-attenuated flu virus. This means it includes a weakened (but not completely dead) version of the virus. In contrast, traditional flu shots contain an inactivated form. Interestingly, the nasal spray vaccine won’t lead to flu illness. According to FluMist’s package insert, the efficacy of the flu spray is comparable to the shot at around 45%, which falls within the range of 40% to 60% efficacy of the injection.
When will you be able to get the at-home FluMist vaccine?
The idea of a self-administered flu vaccine has been simmering for quite some time. However, patience is required, as distribution for at-home use is anticipated for the next flu season, commencing in 2025. Meanwhile, those eager for the nasal spray option can find it already available at a multitude of pharmacies and doctor’s offices—yet, it still necessitates administration by a professional, such as a pharmacist or healthcare provider.
Yahoo Life reached out to FluMist’s manufacturer, AstraZeneca, in search of details about when the product will be available for home use but received no response before press time. One likely reason for this delay is the necessity to gather data on the effectiveness of self-administration among individuals vaccinating themselves and their children, as pointed out by Dr. Davey Smith, chief of infectious diseases and global public health at UC San Diego.
Who should consider using it?
FluMist has received approval for use among individuals aged 2 to 49. “I sincerely hope we can increase vaccination rates, especially with FluMist being authorized for home use, which could benefit children and those who are hesitant about needles,” shares Smith. “Given the myriad barriers in our healthcare system, this will hopefully eliminate at least one hurdle.”
Yet, potential users must fit specific eligibility prerequisites. Neither the FDA, FluMist, nor AstraZeneca have explicitly detailed these standards. However, Smith believes that a questionnaire will likely assess the individual’s age to ensure they are within the ages of 2 to 49 and not on any medications that might have adverse interactions with the vaccine. For instance, children aged 2 to 17 taking aspirin should avoid FluMist or any other live-attenuated vaccines due to their association with a rare yet severe condition known as Reye’s syndrome. Furthermore, pregnant women should also steer clear of the nasal vaccine due to potential risks to the fetus; however, the flu shot is deemed safe and is recommended during pregnancy.
The FDA has also advised that individuals with compromised immune systems and specific allergies should net take FluMist or any live-attenuated virus vaccines. Additionally, its use is not approved for anyone aged 50 or older. Smith elaborates; the injected flu vaccine generates a widespread immune response within the body, while FluMist focuses more locally, which could mean it doesn’t elicit a strong enough response in older adults with a declining immune system.
Are live-attenuated virus vaccines generally safe?
Absolutely, experts assert! The nasal spray contains a compromised but living virus that is “cold-temperature-adapted, allowing it to reproduce itself in the nose—where temperatures are lower than core body warmth,” explains Dr. Paul Offit, director of the Vaccine Education Center at the Children’s Hospital of Philadelphia. “However, it doesn’t replicate in the lungs,” where it could cause an infection.
Associated side effects parallel those linked to the flu shot: a runny nose, nasal congestion, fever in children ages 2 to 6, and sore throat for adults, as detailed by the FDA.
How do you administer FluMist to yourself?
The process resembles that of using an allergy nasal spray like Flonase, as Smith explains—for instance, simply insert the flu nasal spray into one nostril, press the plunger, and then repeat in the opposite nostril. Smith feels confident in the ability of individuals to successfully self-administer the flu spray. “People have been using Flonase for ages; this should be a walk in the park,” he remarks. AstraZeneca’s trial reported that 100% of adults managed to give themselves a full dose.
The straightforward nature of administering the nasal spray vaccine bodes well for children and those averse to needles, as well as public health overall. Offit expresses hope that making FluMist available for home use “should encourage more individuals to receive their flu vaccines,” noting, “There’s no reason this vaccine shouldn’t be delivered intranasally without needing a healthcare professional’s assistance, which is likely to enhance vaccination rates.”
Still, Offit emphasizes the importance of gathering more data on how effectively parents and caregivers are able to administer the spray to their children. “I firmly believe this will elevate vaccination rates,” especially given the recent dip observed since the onset of the COVID-19 pandemic, which saw rates plummet to 50% last year.




